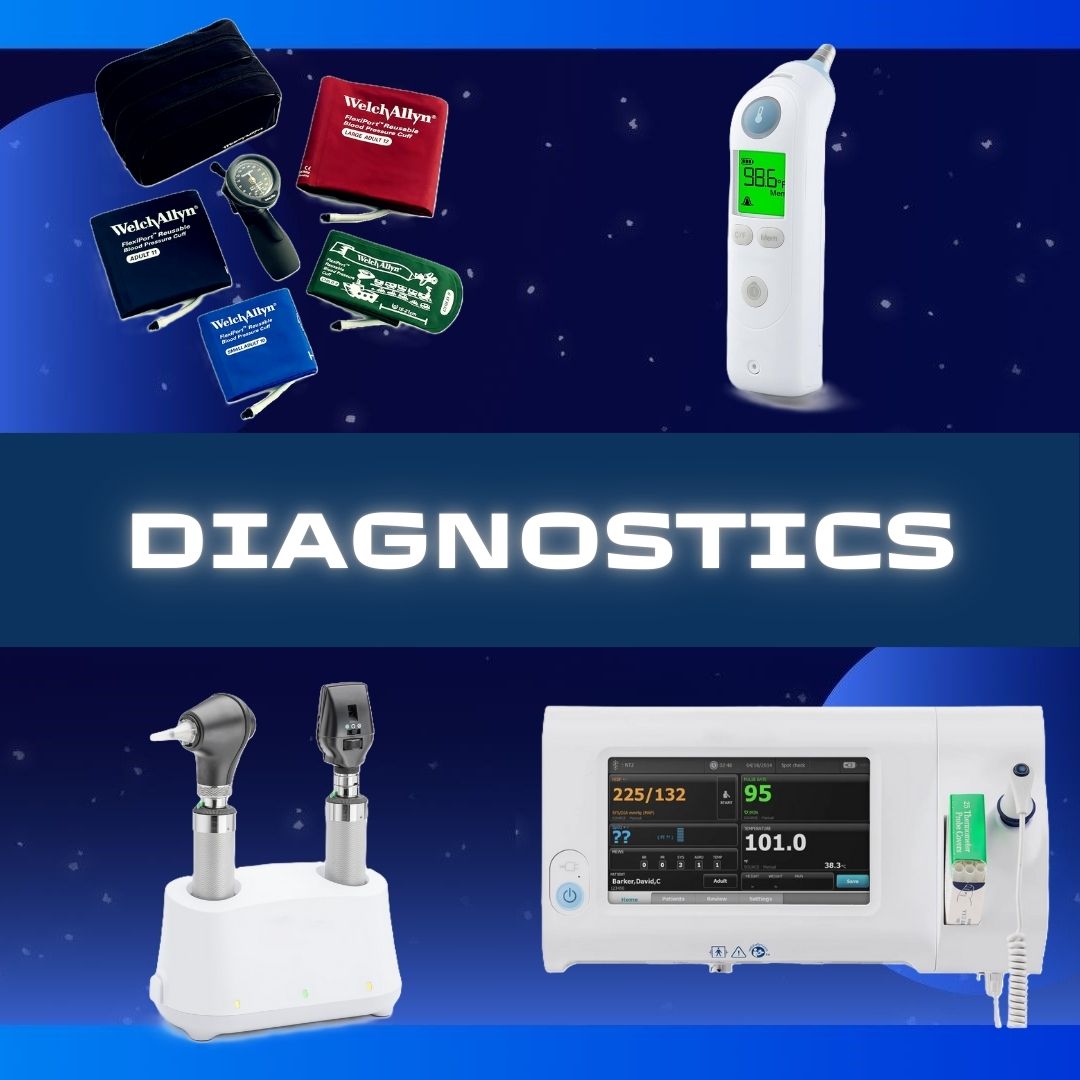
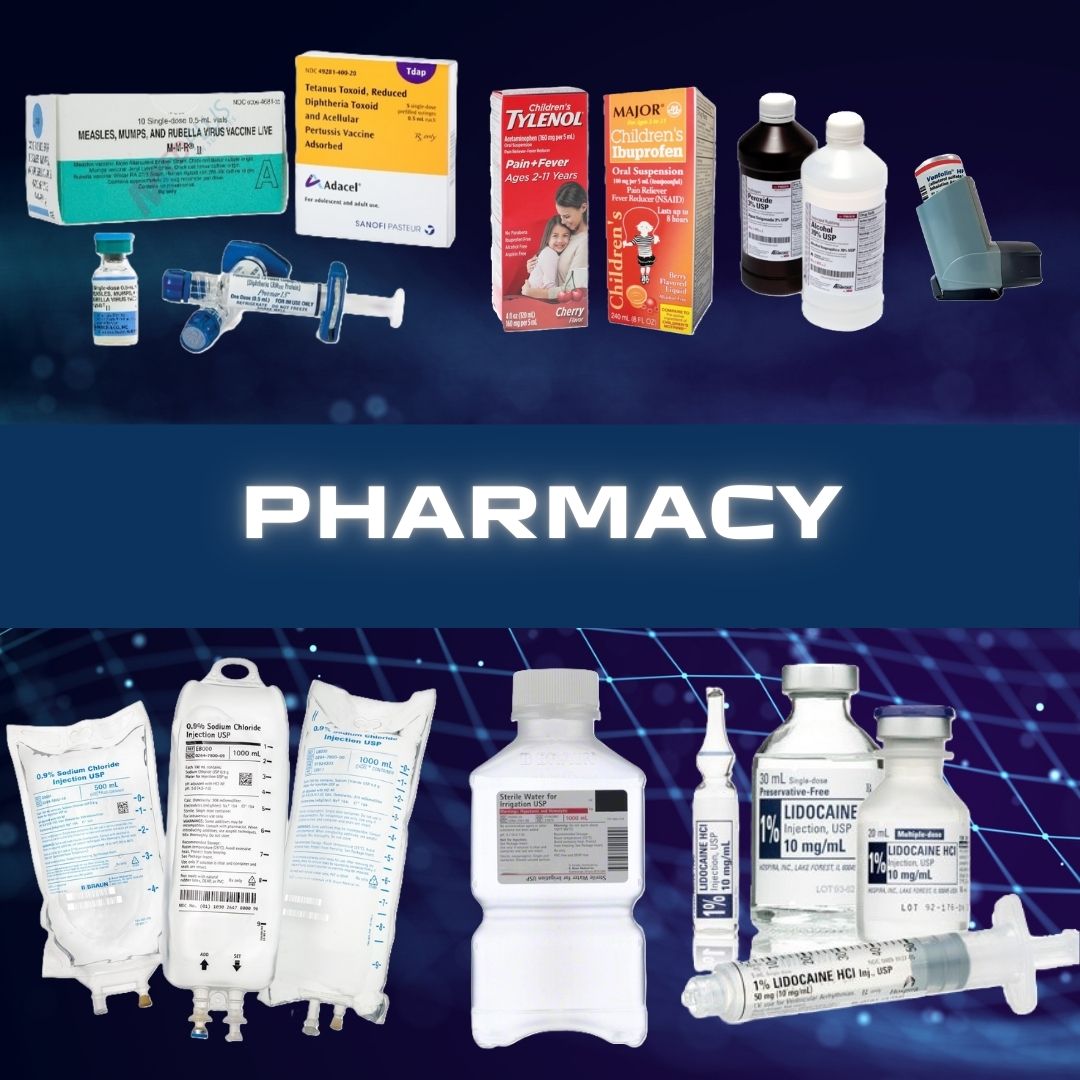
B. Braun #S8004-5264 Replacement Preparation Sodium Chloride, Preservative Free 0.9% Intravenous IV Solution Flexible Bag 100 mL Fill in 150 mL SOD CHL, IVSOL 0.9% PVC-DEHP FREE 100ML/150ML(64/CS) SOLD AS 1 EACH Sodium Chloride Injections USP are sterile, nonpyrogenic, isotonic and contain no bacteriostatic or antimicrobial agents These intravenous solutions are indicated for use in adults and pediatric patients as sources of electrolytes and water for hydration The PAB® container is PVC-Free and DEHP-Free The plastic container is a copolymer of ethylene and propylene formulated and developed for parenteral drugs The copolymer contains no plasticizers and exhibits virtually no leachability The container has two ports, one is for the intravenous administration set and the other is a medication addition site Each is covered by a tamperproof barrier This product is designed for use as a diluent and delivery system for intermittent intravenous administration of compatible drug additives Manufacturer # S8004-5264 Manufacturer B. Braun Country of Origin Unknown Application Replacement Preparation Container Type Flexible Bag Dosage Form IV Solution Generic Drug Code 02962 Generic Drug Name Sodium Chloride, Preservative Free NDC Number 00264-1800-32 Product Dating Acceptable Dating: we will ship >= 90 days Storage Requirements USP Controlled Room Temperature Strength 0.9% Type Intravenous UNSPSC Code 51191602 Volume 100 mL Fill in 150 mL
Size: 100/150ML
More Information