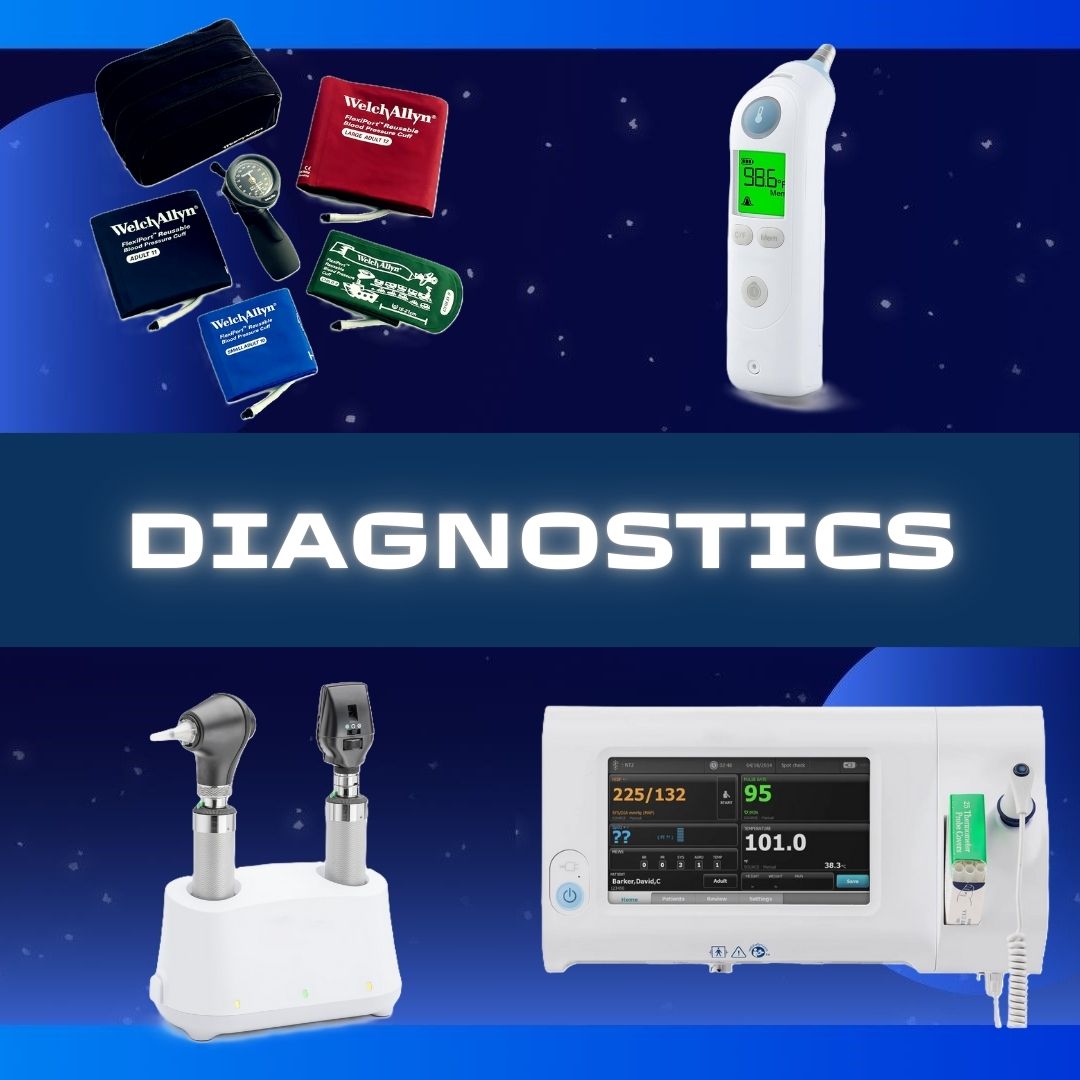
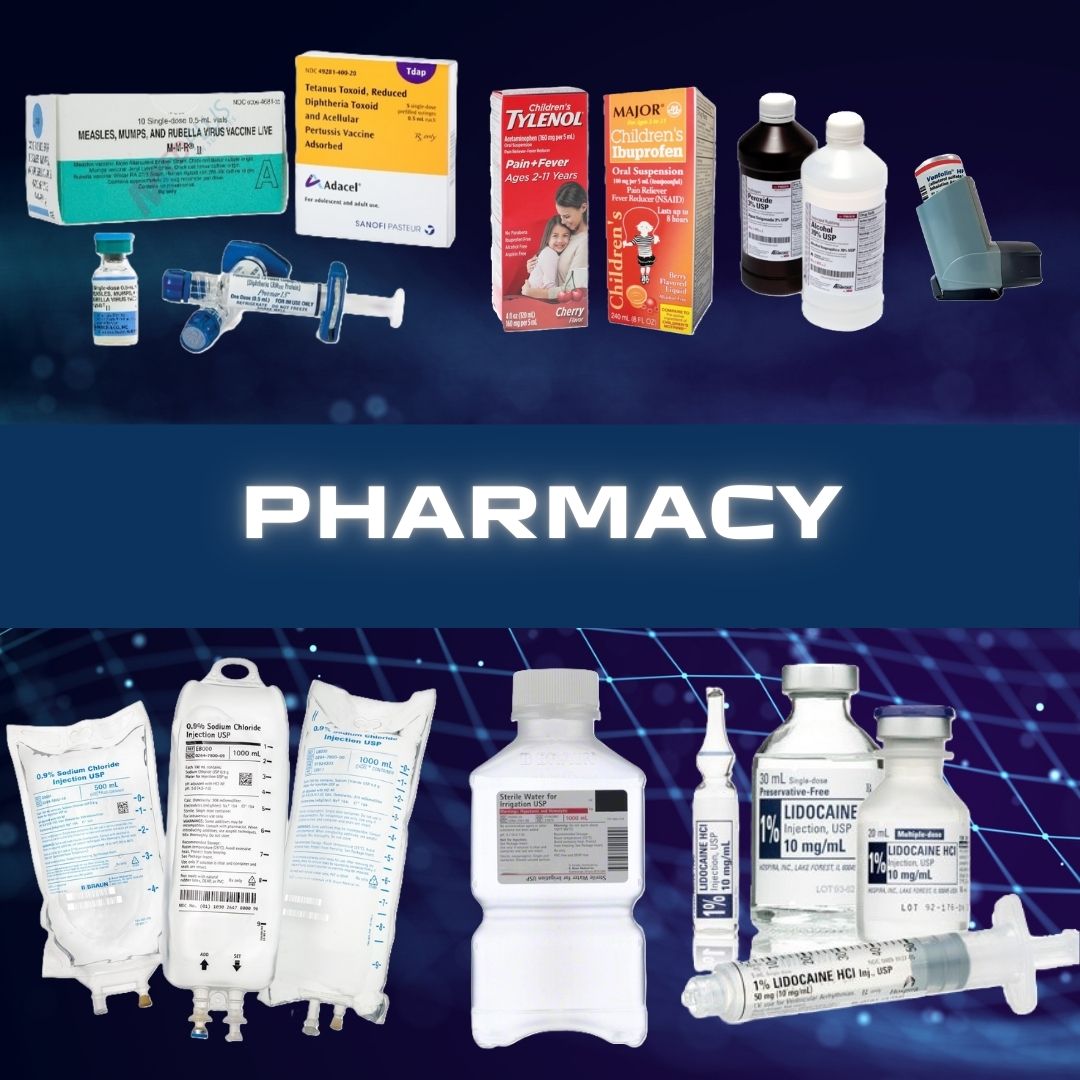
Magellan Diagnostics #70-6760 Blood Lead Analyzer LeadCare® II 1 Test CLIA Waived ANALYZER, BLD LEAD CARE II D/S Features No manual calibration or refrigeration required Point-of-care results in three minutes Requires 2 drop (50 µl) Capillary or venous whole blood (EDTA or Heparin anticoagulated) Includes AC power adapter, Users Guide, Quick Referemce Guide, CDC Blood Collection Video (DVD) and Instructional Video (DVD) For use with LeadCare II Test Kit 70-6762 nd Controls 70-3440 Manufacturer # 70-6760 Brand LeadCare® II Manufacturer Magellan Diagnostics Country of Origin Unknown Application Blood Lead Analyzer CLIA Classification CLIA Waived Contents 1 Analyzer, AC Power Cord, AA Batteries, User's Guide (English), Quick Reference Guide, Instructional Video, and CDC Blood Collection Video Dimensions 3-1/2 X 6-1/2 X 9 Inch For Use With For LeadCare II Test Kit 70-6762 Number of Tests 1 Test Power Source (4) AA Batteries or AC Adapter Readout Type LCD Display Sample Capacity 50 µL Sample Sample Type Capillary Test Name Blood Lead Test Time to Results 3 Minute Results UNSPSC Code 41115807 Weight 2.4 lbs.
More Information