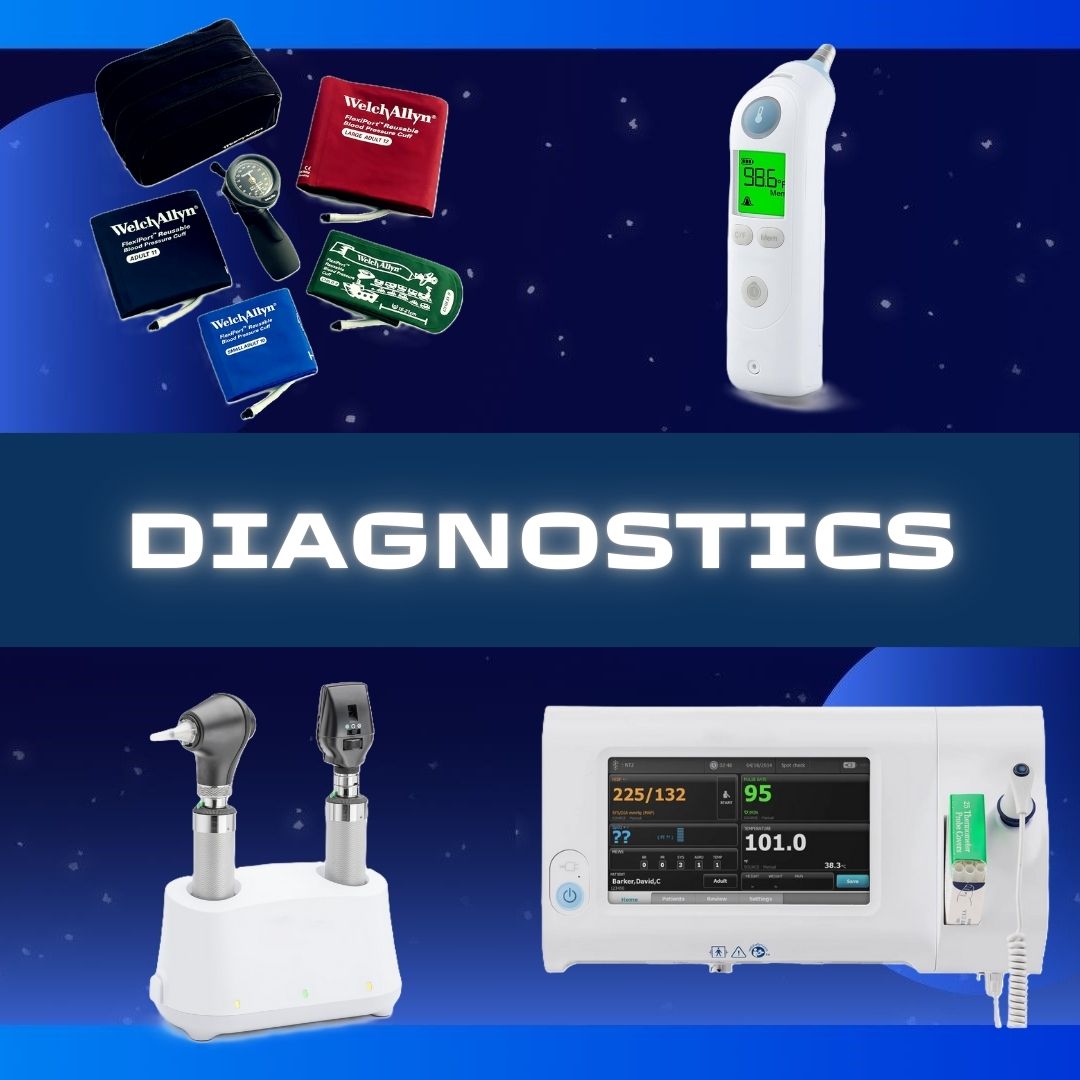
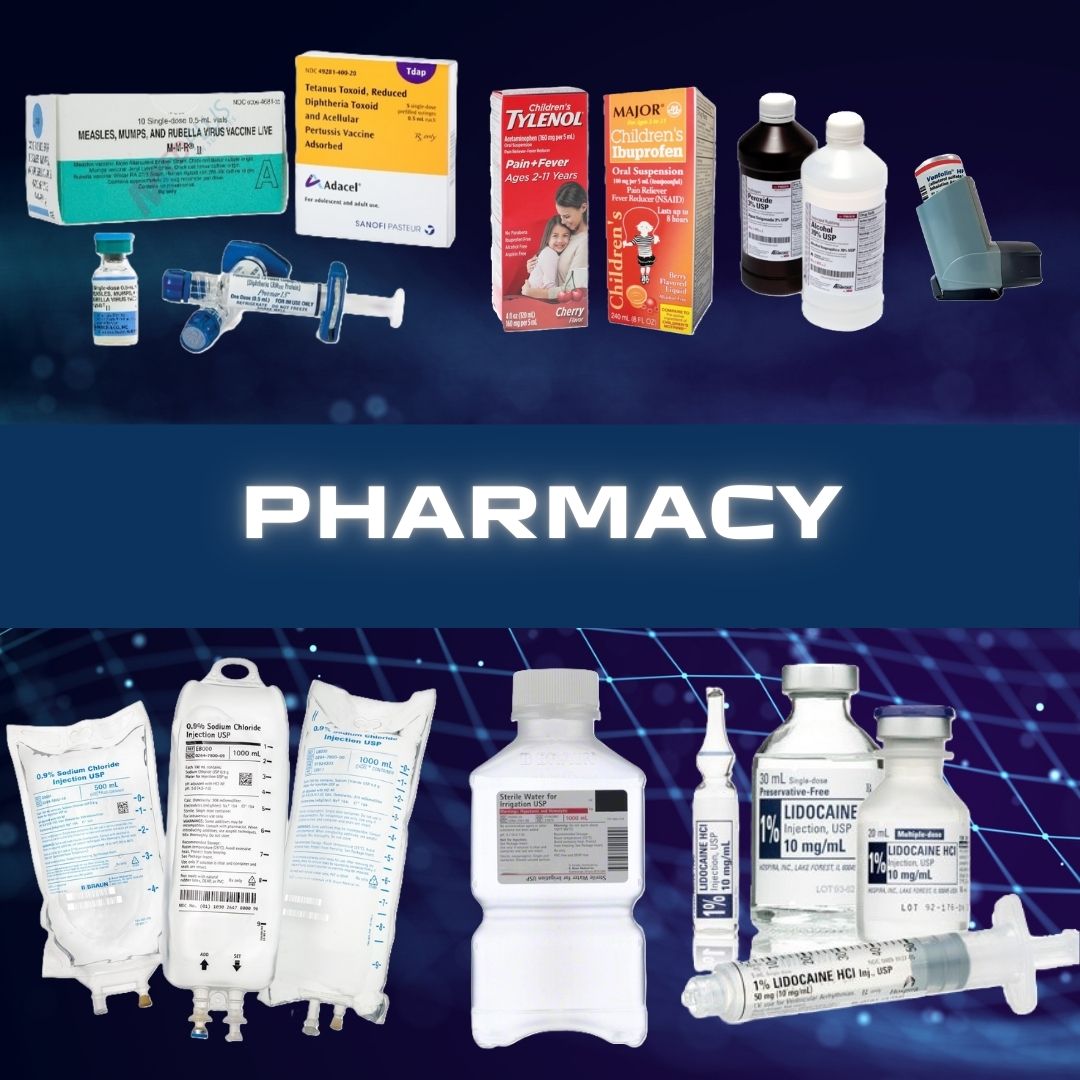
Abbott Rapid Dx North America LLC #427-000 Rapid Test Kit ID NOW™ Influenza A + B 2.0 Molecular Diagnostic Influenza A + B Nasal Swab / Nasopharyngeal Swab Sample 24 Tests TEST KIT, INFLUENZA ALERE NEW AMBIENT (24/KT) Alere I is now ID Now™ Small footprint enables ease of use at point of care Simple operation via visual touchscreen Highly accurate results in 13 minutes Integrated pipette eliminates manual pipetting for direct samples Standardized respiratory trio (Influenza A & B, RSV, Strep) across all sites of care Bi-directional connectivity to industry leading RALS® platform facilitates satellite testing and remote management CLIA COMPLEXITY: WAIVED - For Direct Nasal Swabs (Not Eluted in Viral Transport Media) Only Manufacturer # 427-000 Brand ID NOW™ Influenza A + B 2.0 Manufacturer Abbott Rapid Dx North America LLC Country of Origin United States Application Rapid Test Kit CLIA Classification CLIA Waived Contents 1 Test Bases, Sample Receivers, Transfer Cartridges, Nasal Swabs, Positive and Negative Control Swabs, Plastic Disposable Pipettes, Product Insert and Quick Reference Instructions For Use With For ID Now™ Instrument US Number of Tests 24 Tests Product Dating Acceptable Dating: we will ship >= 90 days Reading Type Machine Read Sample Type Nasal Swab / Nasopharyngeal Swab Sample Test Format Test Device Format Test Name Influenza A + B Test Type Molecular Diagnostic Time to Results 5 Minute Results (Positive), 13 Minute Results (Negative) UNSPSC Code 41116144
More Information