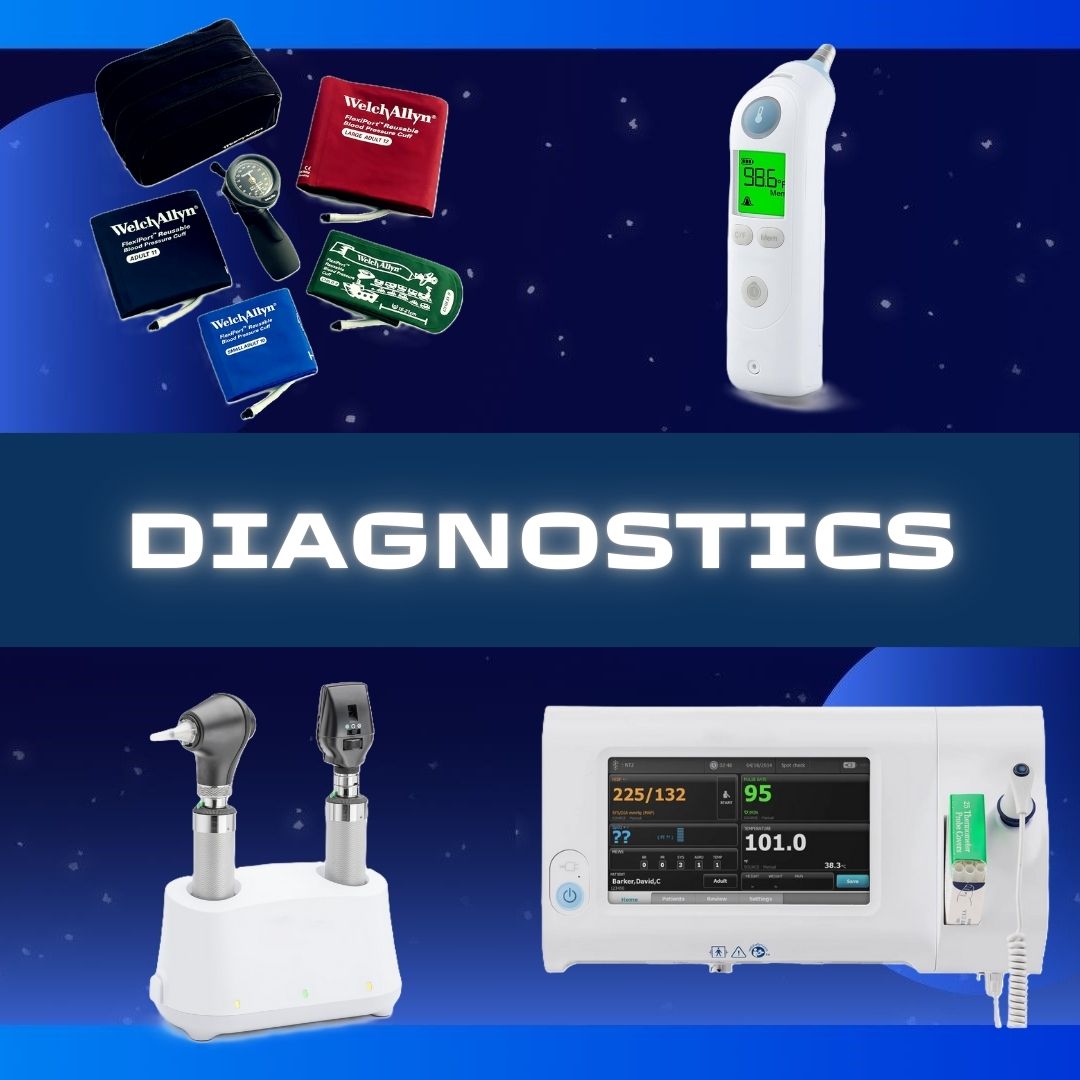
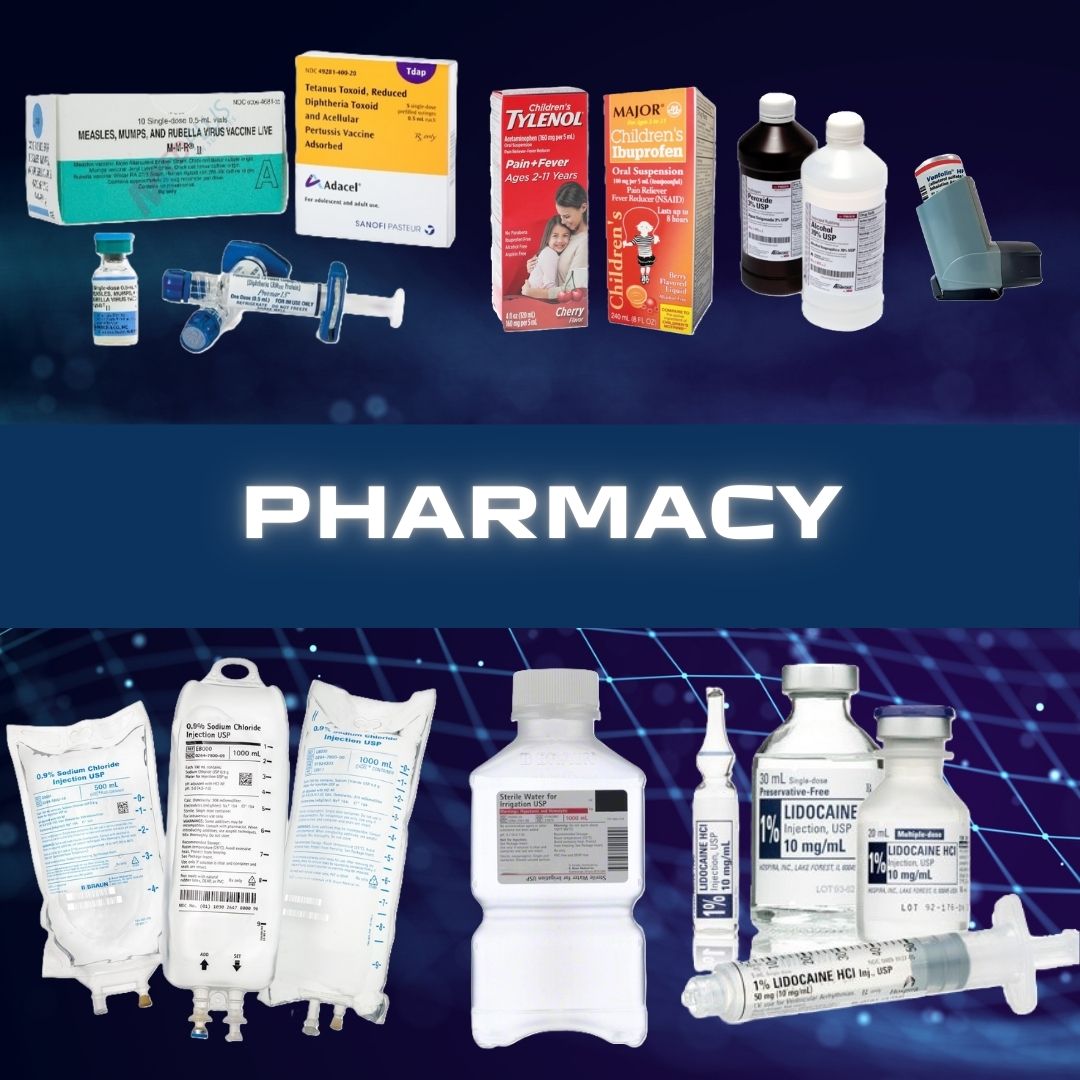
BD Primary Care #256066 Point-of-Care Immunoassay Analyzer BD Veritor™ Plus CLIA Waived READER, BD VERITOR PLUS ANALYZER D/S CLIA-waived digital immunoassay for rapid detection of SARS-CoV-2, Flu A+B, RSV and Group A Strep Advanced particle technology enhances sensitivity by using a proprietary process to produce highly stable, modifi ed colloidal metal particles, helping improve test performance Adaptive read technology helps improve specificity to reduce false-positive results by compensating for background and non-specific binding Fast and accurate assay performance Prefilled color-coded unitized tubes by reagent facilitate workflow Swab or liquid sample is inserted into unitized tube, processed and removed Test device may be inserted immediately into Analyzer (Walk Away mode) or after incubation time is complete (Analyze Now mode) Print-enabled through USB Good for 10,000 tests or 24 months without maintenance or calibration Manufacturer # 256066 Brand BD Veritor™ Plus Manufacturer BD Primary Care Country of Origin Unknown Application Point-of-Care Immunoassay Analyzer CLIA Classification CLIA Waived Contents BD Veritor Plus Analyzer, Instructions for Use Manual, USB Port Unlock Barcode Label, AC Power Adapter, BD Verification Cartridge Dimensions 7.6 X 9 X 14.3 cm For Use With For BD Veritor™ System Test Devices Power Source 100 to 240 VAC at 50 to 60 Hz, 0.6A Readout Type Digital Readout, Printout (Option) Sample Type Throat Swab / Nasal Swab / Nasopharyngeal Swab / Nasopharyngeal Aspirate or Wash Sample Test Name Influenza A + B, Respiratory Syncytial Virus (RSV), and Group A Strep Time to Results 5 Minute Results UNSPSC Code 41115820 User Interface LCD Display Weight 300 g
BD Primary Care #256066 Point-of-Care Immunoassay Analyzer BD Veritor™ Plus CLIA Waived READER, BD VERITOR PLUS ANALYZER D/S CLIA-waived digital immunoassay for rapid detection of SARS-CoV-2, Flu A+B, RSV and Group A Strep Advanced particle technology enhances sensitivity by using a proprietary process to produce highly stable, modifi ed colloidal metal particles, helping improve test performance Adaptive read technology helps improve specificity to reduce false-positive results by compensating for background and non-specific binding Fast and accurate assay performance Prefilled color-coded unitized tubes by reagent facilitate workflow Swab or liquid sample is inserted into unitized tube, processed and removed Test device may be inserted immediately into Analyzer (Walk Away mode) or after incubation time is complete (Analyze Now mode) Print-enabled through USB Good for 10,000 tests or 24 months without maintenance or calibration Manufacturer # 256066 Brand BD Veritor™ Plus Manufacturer BD Primary Care Country of Origin Unknown Application Point-of-Care Immunoassay Analyzer CLIA Classification CLIA Waived Contents BD Veritor Plus Analyzer, Instructions for Use Manual, USB Port Unlock Barcode Label, AC Power Adapter, BD Verification Cartridge Dimensions 7.6 X 9 X 14.3 cm For Use With For BD Veritor™ System Test Devices Power Source 100 to 240 VAC at 50 to 60 Hz, 0.6A Readout Type Digital Readout, Printout (Option) Sample Type Throat Swab / Nasal Swab / Nasopharyngeal Swab / Nasopharyngeal Aspirate or Wash Sample Test Name Influenza A + B, Respiratory Syncytial Virus (RSV), and Group A Strep Time to Results 5 Minute Results UNSPSC Code 41115820 User Interface LCD Display Weight 300 g