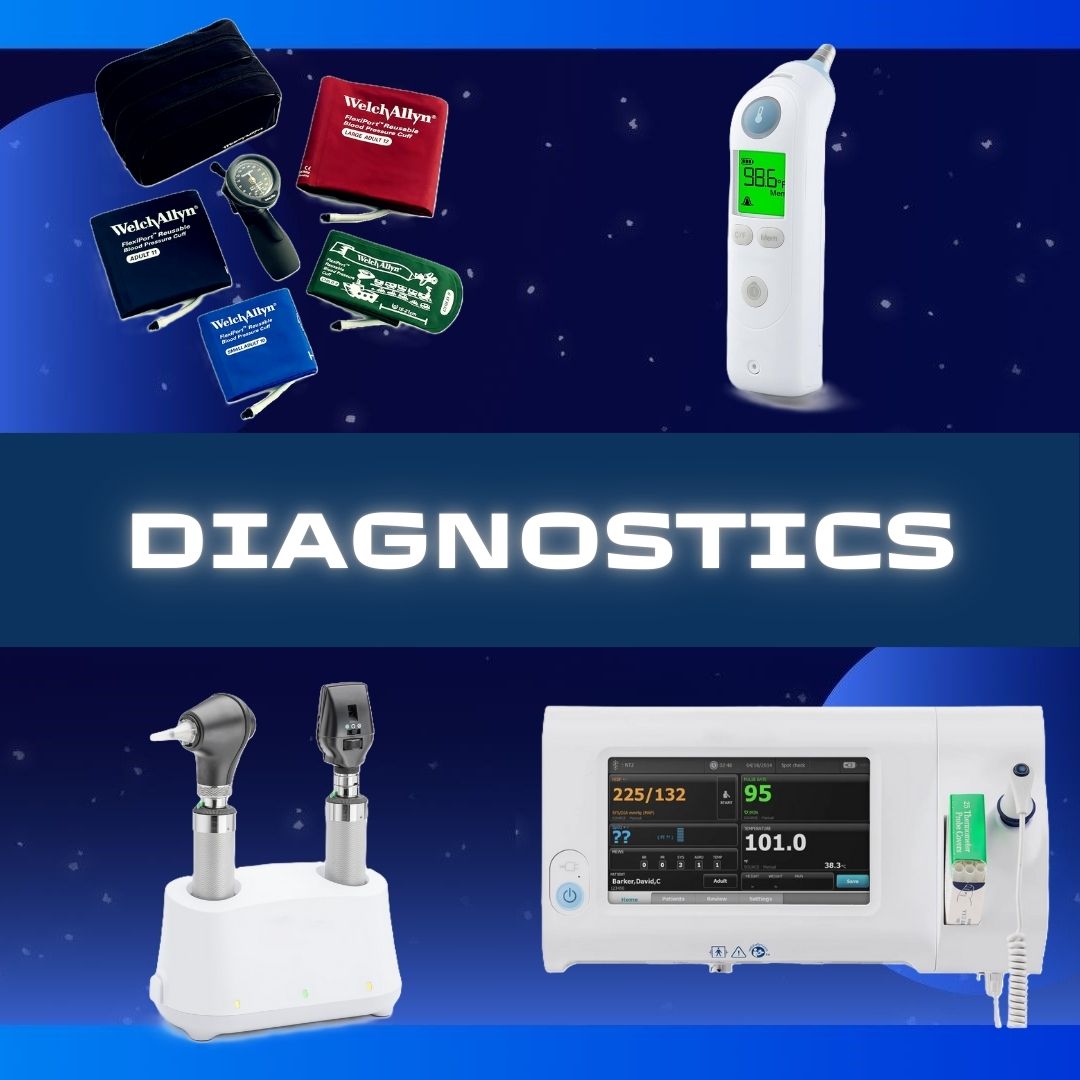
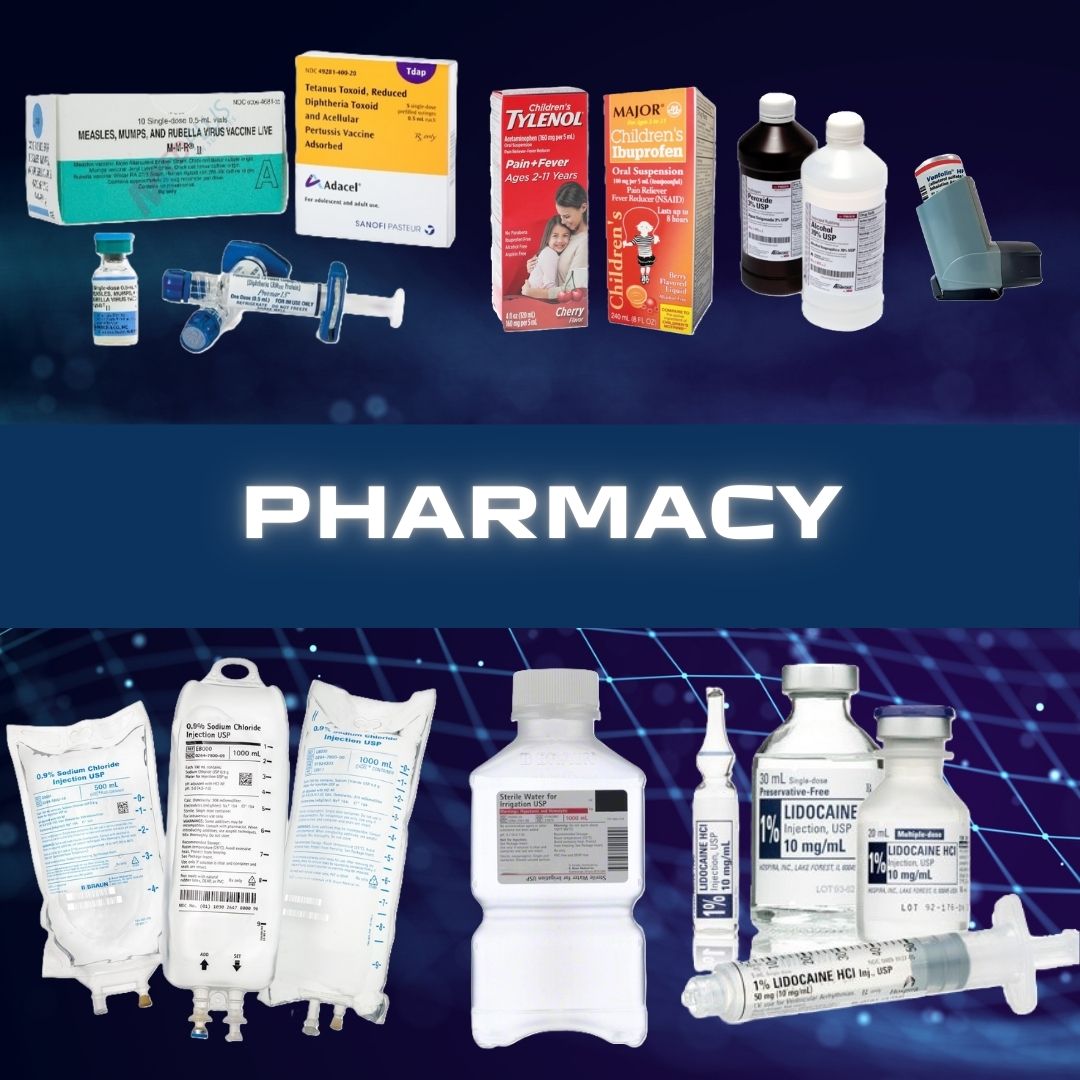
Abbott Rapid Dx North America LLC #430122 Rapid Test Kit BinaxNOW® Infectious Disease Immunoassay Respiratory Syncytial Virus Test (RSV) Nasopharyngeal Swab / Nasal Wash Sample 22 Tests TEST KIT, WAIVED BINAX NOW RSV22 (22/KT) WAMPOLE Alere BinaxNOW® RSV Card is an in vitro rapid immunochromatographic assay used to detect respiratory syncytial virus (RSV) fusion protein antigen in nasal wash and nasopharyngeal (NP) swab specimens from symptomatic patients It is intended to aid in the diagnosis of RSV infections in patients under the age of 5 Retrospective nasal wash Sensitivity: 89% Specificity: 100% Prospective NP swab Sensitivity: 93% Specificity 93% Manufacturer # 430122 Brand Binaxnow® Manufacturer Abbott Rapid Dx North America LLC Country of Origin United States Application Rapid Test Kit CLIA Classification CLIA Waived Contents (22) Test Cards, Transfer Pipettes, Positive Control Swab, Negative Control Swab, Elution Solution, NP Patient Swabs, Product Insert, Procedure Card Number of Tests 22 Tests Product Dating Acceptable Dating: we will ship >= 90 days Reading Type Visual Read Sample Type Nasopharyngeal Swab / Nasal Wash Sample Test Format Test Card Format Test Method Lateral Flow Method Test Name Respiratory Syncytial Virus Test (RSV) Test Type Infectious Disease Immunoassay Time To Results 15 Minute Results UNSPSC Code 41116144
More Information