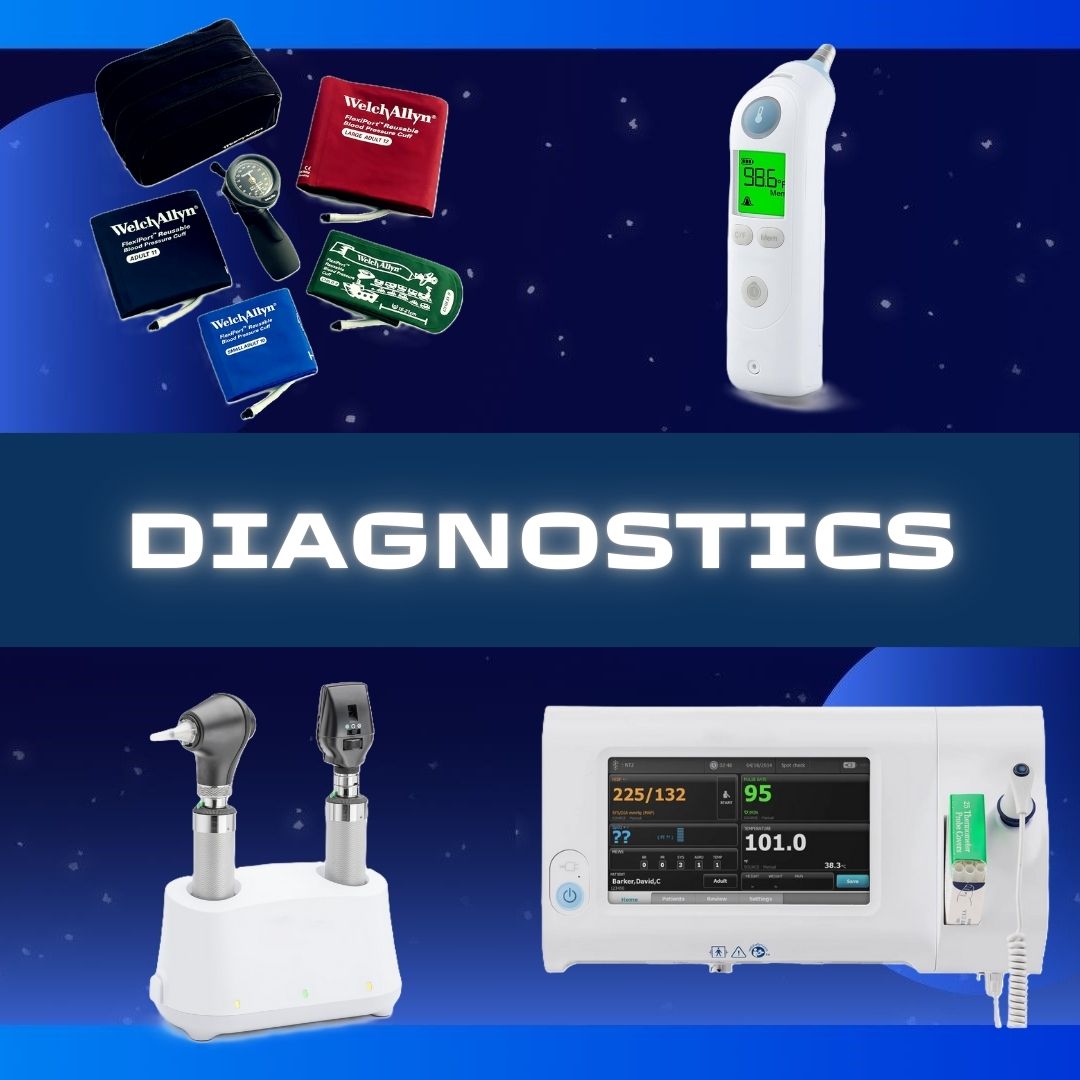
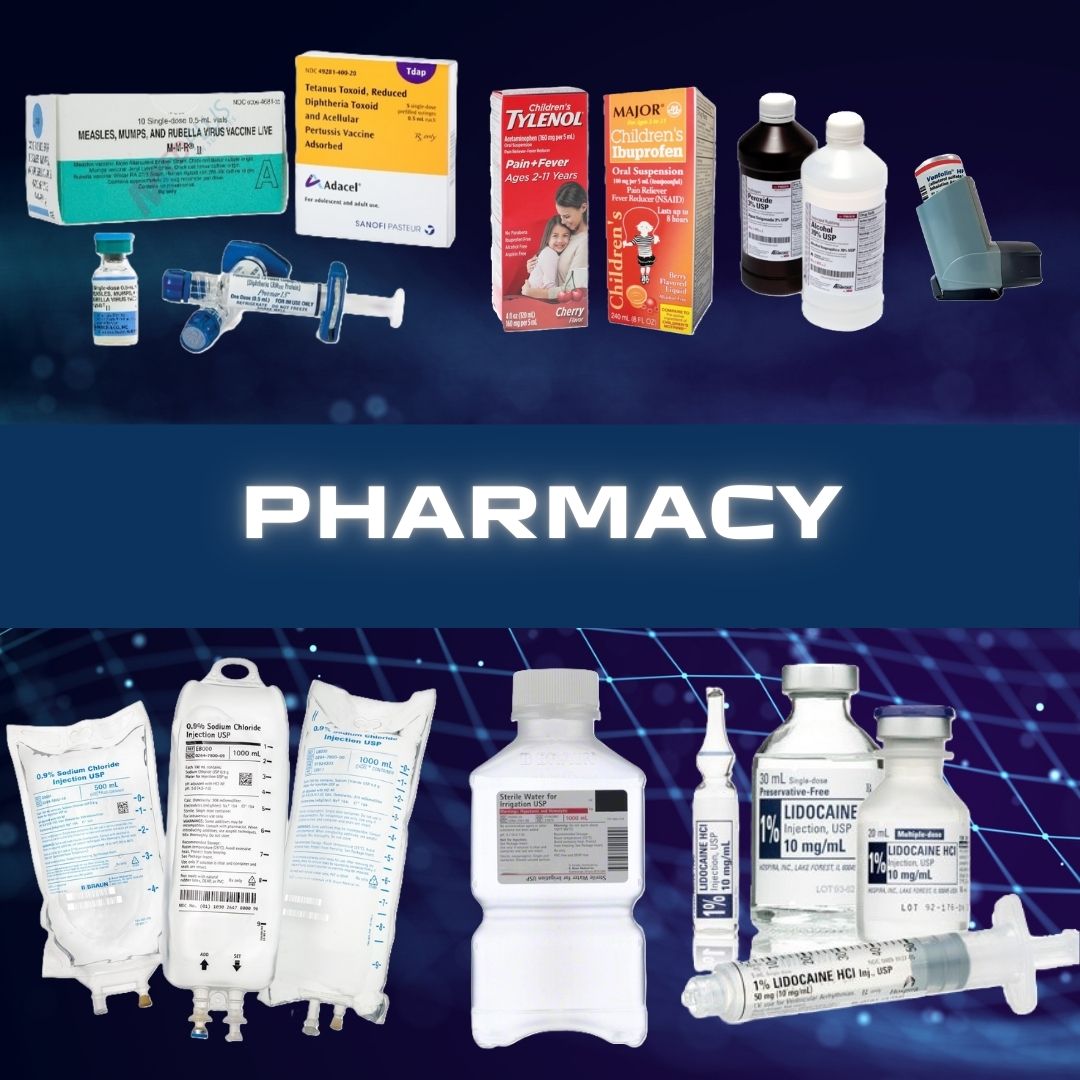
Mini Cart
Your Cart Is Empty
Top Sellers
Set I.V. Administration 15 Drops / mL Drip Rate 78 Inch Tubing 1 Port Latex free Y-injection site Sterile (1/EA 50/CS) by Exel Corporation ID 29081
DetailsGloves Exam ProAdvantage® Medium NonSterile Latex Standard Cuff Length Textured Fingertips Ivory Not Chemo Approved (100/BX 10BXS/CS) by Pro Advantage ID P359103
US$7.61 DetailsDrape Sheet General Purpose ProAdvantage Physical Exam Drape 2-Ply White 40 W X 48 L Inch NonSterile (100/CS 60 CS/PLT) by Pro Advantage ID P754048
US$25.68 DetailsElectrode ECG 3M™ Red Dot™ Resting Radiolucent 100 per Pack (100/BG 40BG/CS) by Solventum Corporation ID 2360
DetailsGloves Exam ProAdvantage® Small NonSterile Latex Standard Cuff Length Textured Fingertips Ivory Not Chemo Approved (100/BX 10BXS/CS) by Pro Advantage ID P359102
US$7.61 Details